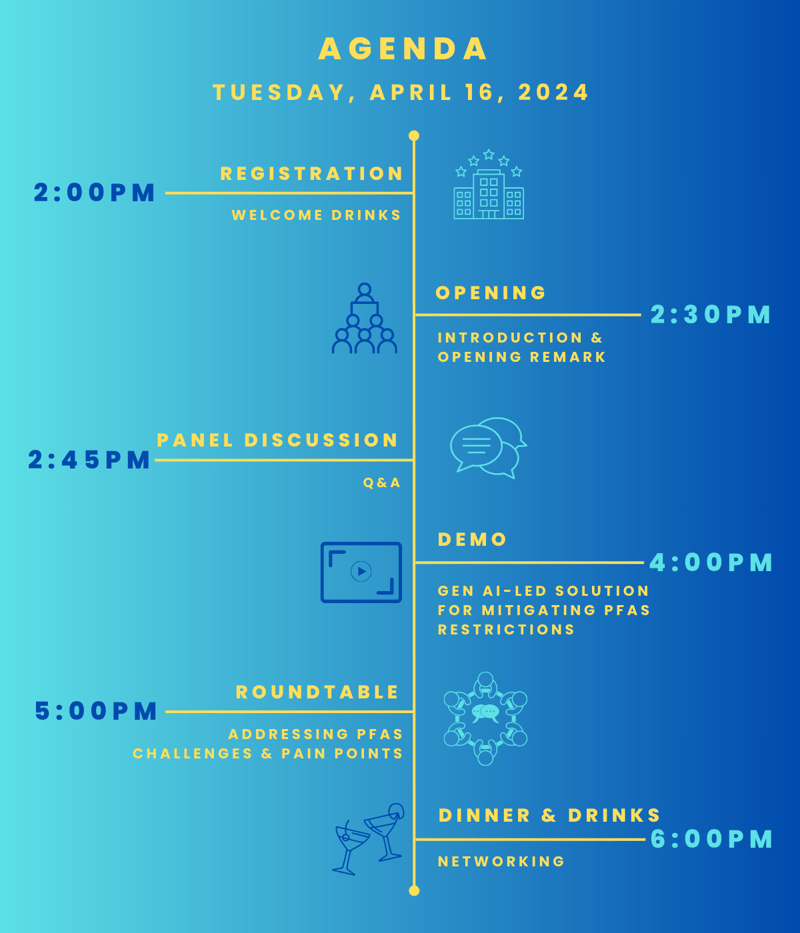
A Tata Elxsi Symposium and Networking Event
Navigating PFAS Restrictions and Regulations in MedTech
Ensuring Business Continuity with Proactive PFAS Compliance
16th April 2024 | 2:00 PM (CET) | Tuttlingen, Germany
The medical device industry confronts new regulatory hurdles with proposed restrictions by the European Chemicals Agency (ECHA) and reporting obligations from the US Environmental Protection Agency (EPA) concerning PFAS usage. As these regulations approach, companies must swiftly adapt to ensure compliance while maintaining product continuity in the market. At Tata Elxsi, we are at the forefront of addressing these challenges, offering tailored solutions to ensure seamless compliance and sustained market presence.
Join us for an exclusive networking event in Tuttlingen, Germany, and learn from the distinguished leaders from the medical device industry. Together, we will tackle the complexities of PFAS regulations and their implications for medical device manufacturers, explore innovative strategies, gain valuable insights, and foster meaningful connections with industry peers.
With only a few slots left, ensure your participation by registering now!
Delegate Registration
Panel Discussion
Panel Moderator

Dolly Sharma
Practice Lead, Regulatory Compliance,
Tata Elxsi (Ex-TUV SUD Auditor)
Innovation in Compliance Amidst PFAS Restriction Regulations
- Exploring cutting-edge approaches to maintain compliance while navigating the intricacies of PFAS regulations.
- Addressing the ethical considerations and challenges inherent in regulatory compliance.
- Learning how industry collaboration and regulatory engagement can drive progress within regulatory boundaries.
Key Focus
Demonstration
Gen AI-led solution for mitigating PFAS restrictions

Roundtable
Collaborative Strategies for a Successful PFAS Restriction Mitigation Plan
Who should attend?
This event welcomes individuals from the medical device industry, spanning roles such as CXOs, Vice Presidents, and Directors in the domains of QARA (Quality Assurance and Regulatory Affairs), Biocompatibility, R&D, and Product Stewardship.