Case Study
EU MDR Remediation and Global Submission Support for Non-EU Countries

Countries Targeted for Dossier Submission
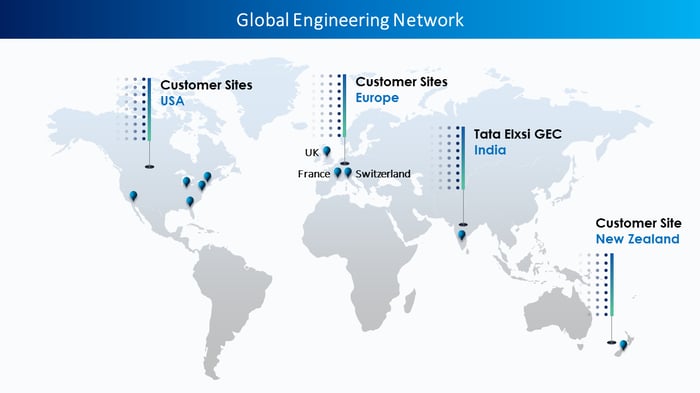
Customer Sites Supported
Products Supported
Background
Our client is a US-headquartered global leader in medical technology, services, and solutions with a presence in over 150 countries and more than 350 locations. They specialize in various therapy areas such as Cardiovascular, Cardiac Rhythm, Digestive & Gastrointestinal, and Diabetes, to name a few.
The client intended to address the impact of EU MDR in non-EU countries to ensure compliance and sustain market access beyond the European Union.
Challenge
The EU Medical Device Regulations (MDR) not only impacted the compliance requirements in the EU region but also made regulatory compliance more stringent in the rest of the world (ROW), particularly in countries mandating CE mark for medical device approval. Therefore, navigating complex regulatory requirements and adapting to different compliance standards while also addressing diverse cultural and linguistic considerations was a major challenge.
Additionally, the customer had multiple business units with inconsistent processes and systems across all manufacturing and vendor sites. Managing legacy products proved particularly cumbersome, leading to a backlog affecting 128+ product families, further intensifying the challenge.
Solution
Tata Elxsi established cross-functional communication within the client organization and their network of contract manufacturers and vendors across geographies for upfront data collection and regulatory impact assessment. We prepared a global submission dossier template and country-specific database to streamline the entire workflow and process.
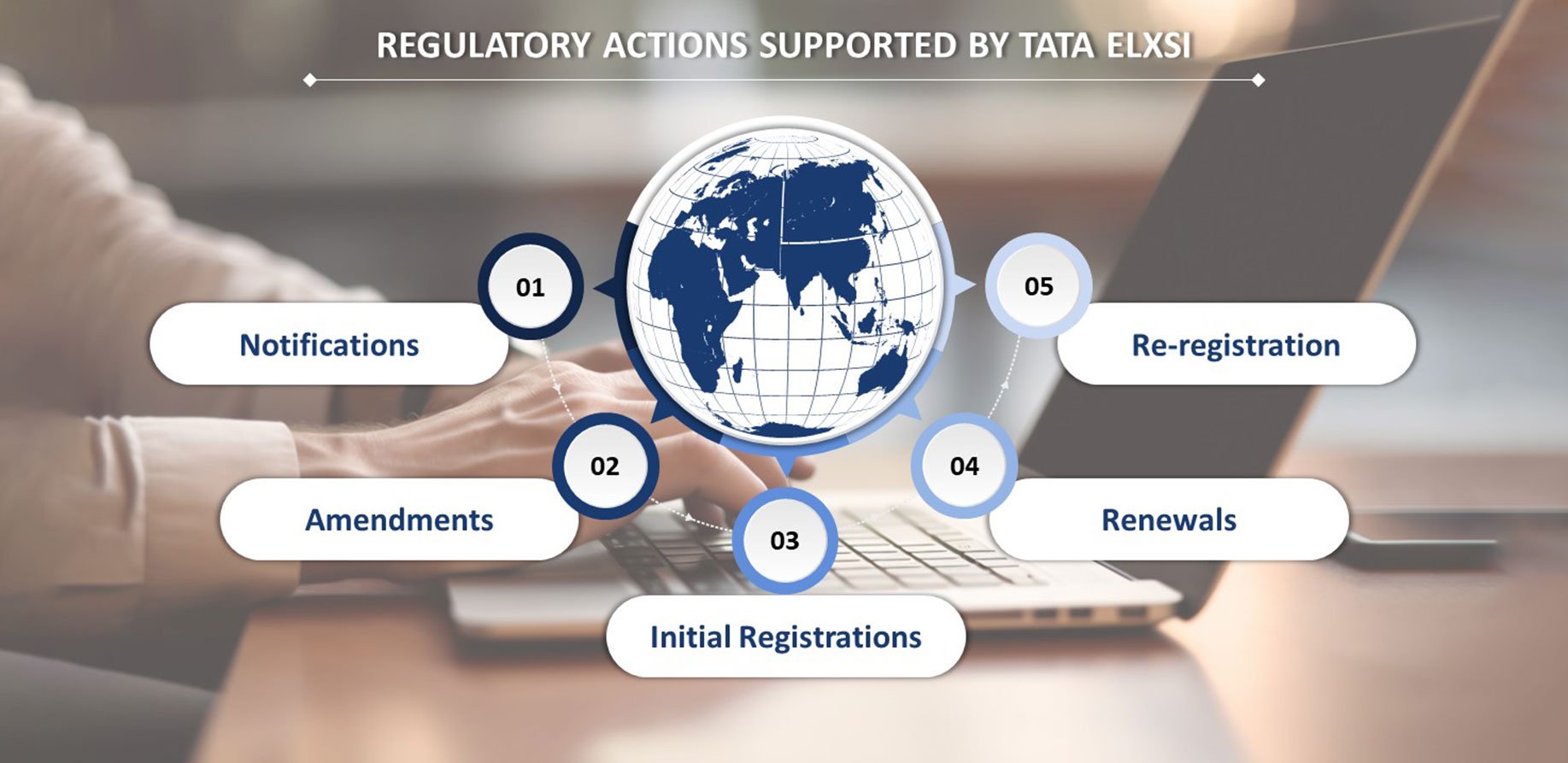
After a thorough regulatory assessment, we created a list of documents needed for each country. We fetched the necessary files from the PLM tool and crafted a submission-ready dossier. We also supported the customer throughout the submission and review process and developed a tracker to monitor approvals.
Impact
- Established a dedicated Global Engineering Center (GEC) with a team of cross-functional specialists to develop submission dossiers for country-specific requirements.
- Ensured uninterrupted market access to 67+ countries.
- Implemented an effective tracking mechanism and adopted agile processes in program management, reducing the lead time by 30%.
- Developed a regulatory playbook, meticulously documenting critical processes and country-specific requirements. Also, created and delivered training modules to empower the client's team.
Scope of work
Tata Elxsi
- EU MDR Change Analysis
- Regulatory Impact Analysis
- STED Preparation/ Update
- Country-specific Dossier Preparation
- Submission and Approval Tracking