Tata Elxsi at American Medical Device Summit 2023
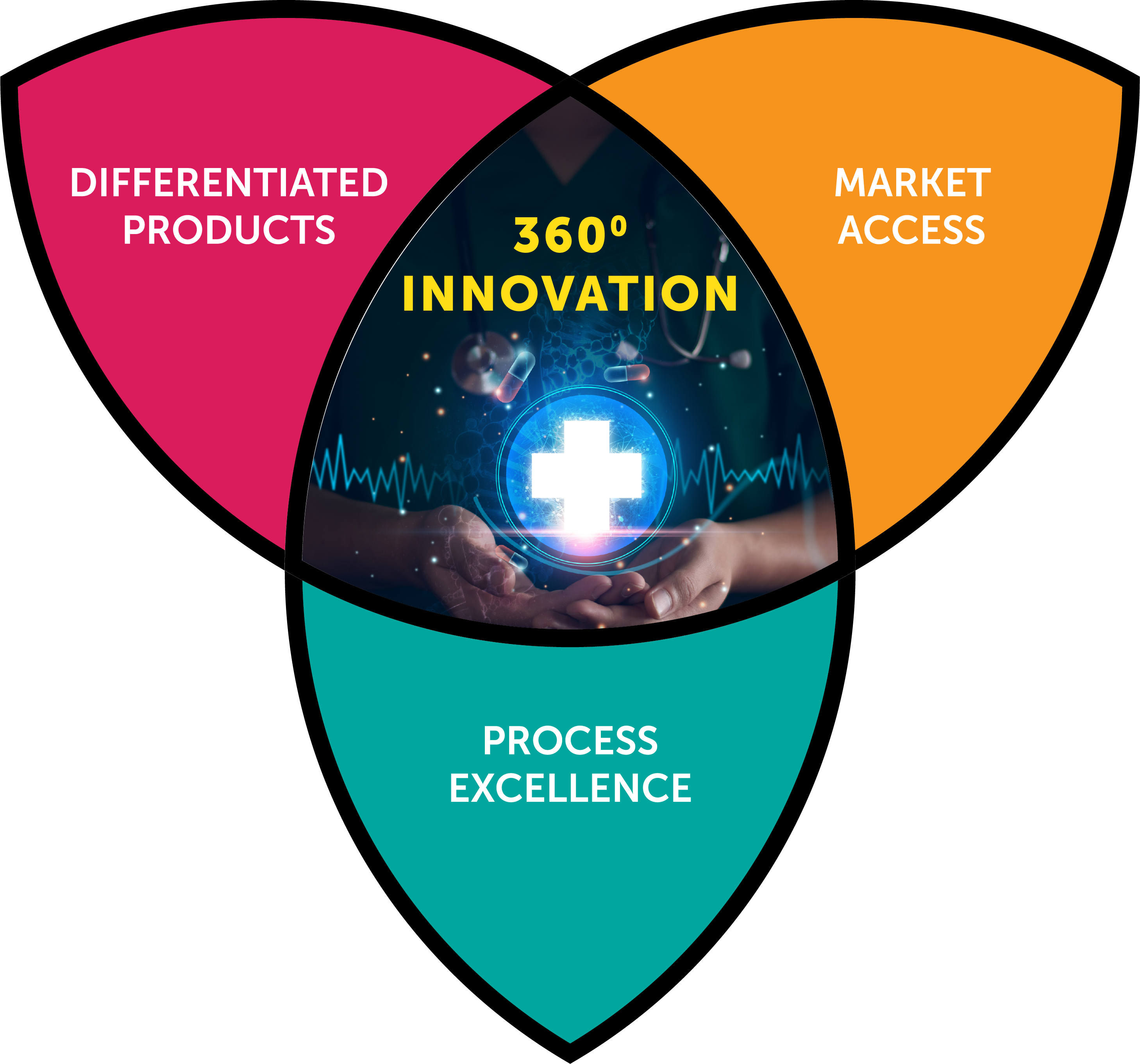
Inspiring Success with 360º Innovation
26th & 27th September 2023, Delegate Lounge, Westin Chicago Lombard, Chicago
Tata Elxsi is excited to be a “Premier Industry Partner” at the 10th Anniversary of the American Medical Device Summit 2023!
Meet our team to learn how we are helping medical device businesses conceptualize, transform, commercialize, and sustain their products in more than 67 countries globally. Together, let's chart a transformative course, creating not just solutions, but impactful journeys.
Join us in Chicago to exchange technology and industry insights and embrace the 360º spectrum of innovation with us.
Get a VIP Discount Code
Thought Leadership
Plenary Session
%20(1).png?width=500&height=200&name=Untitled%20(500%20%C3%97%20200%20px)%20(1).png)
Peter Galen, Chief Innovation Officer, Hemex Health, and Muthusamy Selvaraj, VP, Innovation & Emerging Business, Tata Elxsi will speak on "Driving Innovation and Accelerating Patient-Centricity in the Global Medical Device Landscape"
9:20 am – 9:55 am CST | TUESDAY, 26th September 2023
Lunch & Learn Session

Monali Bhansali, Practice Manager, Regulatory Compliance will moderate the roundtable on "Driving Growth and Resilience: Mastering M&A Dynamics in the Evolving MedTech Landscape"
1:05 pm – 2:05 pm CST | TUESDAY, 26th September 2023
Products and Services

Medical Device Engineering
Designing superior and differentiated products with end-to-end medical device design and development expertise.

Regulatory Compliance
Navigate complex regulatory landscape with confidence and certainty with our comprehensive Quality Assurance and Regulatory Affairs (QARA) services.

Digital Health Engineering
Experience the convergence of futuristic medical and digital technologies with our patient-centric digital health engineering services.

User Experience Design
Design intuitive and seamless experiences to enhance user engagement and ensure positive clinical and business outcomes.
Drawing on over two decades of industry expertise in medical device regulatory compliance, product engineering, and industrial design, our ISO 13485:2016 compliant QMS seamlessly aligns with diverse regulatory standards, and Centers of Excellence for AI, IoT, and Extended Reality converge to empower your journey with innovation.
Want to accelerate your medical device transformation journey?
